|
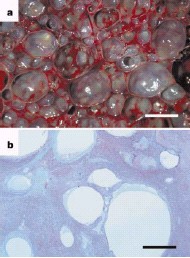
Nitrogen bubbles and emboli can develop in decompression sickness in humans and experimental animals as a result of expansion of pre-existing gas nuclei within nitrogen-supersaturated tissues5. Anatomical, physiological and behavioural adaptations may mitigate against in vivo formation of nitrogen bubbles in marine mammals8, although there is empirical evidence of nitrogen supersaturation in cetaceans8.
Some deep-diving species are predicted to undergo up to 300% nitrogen tissue supersaturation7.
Static diffusion in nitrogen-supersaturated tissues is therefore a plausible mechanism for bubble development and is consistent with a greater prevalence of cases in deep-diving species such as Risso’s dolphins and beaked whales.
Further investigation is needed into the physical and behavioural effects on cetaceans exposed to sonar, and the relation of these effects to bubble growth in vivo and to strandings. Necropsies should aim to compare fat and gas emboli in stranded cetaceans suspected of having been exposed to sonar with results from unexposed stranded controls. In a wider conservation sense, our findings need to be taken into account in considering the regulation and limitation of the adverse impact of anthropogenic sonar on cetaceans.
P.D. Jepson*, M.Arbelo†, R. Deaville*, I. A. P. Patterson‡, P. Castro†, J. R. Baker§, E. Degollada†, H.M. Ross‡, P.Herráez†, A. M. Pocknell*, F. Rodríguez†, F. E.Howie||, A. Espinosa†, R. J. Reid‡, J. R. Jaber†, V. Martin†, A. A. Cunningham*, A. Fernández†
* Institute of Zoology, Zoological Society of London,
Regent’s Park, London NW1 4RY, UK
† Histology and Pathology Unit, Institute for
Animal Health, Veterinary School, Montana
Cardones-Arucas, University of Las Palmas de
Gran Canaria, Gran Canaria, Spain
e-mail: afernandez@dmor.ulpgc.es
‡ Wildlife Unit, SAC Veterinary Science Division
(Inverness), Drummondhill, Stratherrick Road,
Inverness, IV2 4JZ, UK
§ Department of Veterinary Pathology, University of
Liverpool, Leahurst, Neston,Wirral CH64 7TE, UK
|| SAC Veterinary Science Division (Edinburgh),
Allan Watt Building, Bush Estate, Penicuik,
|